THINGS THAT MATTER
Take a look around yourself, what do you see? A table, cell phone, glass, water, wood, a pen, a car, and the list go on. We see so many different things around us, all different in their working, property, material. Although all of us have studied this in our science classes we never really take a moment to think that all that we see around ourselves, even we, the stars in the sky, are all made of the basic building blocks – atoms.
 |
| (Photo : Google pics) |
But the question is how can one particular thing be the basic building block of everything? All the things we see around us are completely different than the other. For example, if you drop your cell phone it won’t wet the surface like water does, you certainly cannot travel great distances sitting on a table like a car, and clearly we humans do not glow like the stars in the sky. So what exactly is going on? The behaviours of things we see around us depend on how atoms are arranged inside elements, what element is the object made of, how they interact with each other, etc.
To understand this better let us talk about – atoms.
Atoms, as we know, are the basic building blocks of everything. Atoms, in turn, are made out of – protons, electrons and neutrons. The arrangement of the protons, electrons and neutrons is what makes the difference in elements. For example, take one proton and make one electron spin around the proton and you would have created the very first element – hydrogen, which in fact is the most abundant chemical substance in the entire universe. Take another proton, two neutrons and stick them closely with the previous proton and make another electron spin around them. There you have – helium, the second most abundant substance of the universe. Add 6 more protons, electrons and neutrons to the structure to create – oxygen. Just like this, all different elements are created from these 3 basic particles, hence giving every element their own unique characteristics.
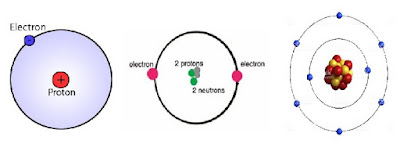 |
| (Photo : Google pics) |
These elements, in turn, combine to form molecules and molecules combine to form large complex chemical structures which in turn form everything we see around us. That is how the world we see around us came into existence. According to the physical structure, the atom has a central nucleus which is made up of protons and neutrons and the electrons orbit around the nucleus. Now atoms are so small that you will need something called an electron microscope to see them. The size of the nucleus is much smaller than the atom itself. For example, the size of the helium atom is 1 Angstrom i.e. 10-10 meters and the nucleus is 1 femtometer in diameter i.e. 10-15 meters. But how small is that? Consider this, if you think of yourself as the nucleus of helium the electrons orbit will be 100 km away from you. The 100 km in between is completely empty. This, in fact, is an important fact – that the atom is mostly empty. This was proven in the famous gold foil experiment by Ernest Rutherford.
 |
| (Photo : Google pics) |
But what is the need to understand the atom? What good does it do? As we saw atoms form the abundance of matter and substance we see around us. So understanding the properties of the elements is very important. Understanding things at the most fundamental level help us improve our modern technology, like in the fields of particle physics, molecular biology, chemistry, nano-computing, quantum computing, nuclear power management and much more. All of this to understand things better and utilize that knowledge for the betterment of mankind.
~ Devraj Roy
Previous Article :
Follow us on :
Facebook page :
Instagram :
Twitter :





Comments
Post a Comment